Abstract
Outcome in younger patients (pts) with ALL has been improved considerably using intensive pediatric-based therapy, but limited data on this approach have been reported for older pts and even the age cut-off is heavily debated. The German Multicenter Center Study Group for Adult ALL (GMALL) has conducted a clinical trial (>55 years) (NCT00198978) which was followed by a registry trial based on standard management recommendations with prospective documentation in the GMALL registry (NCT02872987). Strategies were modified over the years (yrs). The backbone included: Pre-phase (Dexa, Cyclo), induction I (Dexa, VCR, Idarubicine), induction II (Cyclo, AraC), ±post-induction PEG-ASP, consolidation (C) cycles with IDMTX (± E.coli ASP), HDAraC (earlier: VM26), reinduction (VCR, Idarubicine, Cyclo, AraC), ± Rituximab in CD20+, i.th. prophylaxis and maintenance (6-MP/MTX) (group 1). The latest protocol (from 2017) additionally included IDMTX/PEG-ASP in consolidation and recommended MRD-based treatment modification (Blinatumomab in B-Lin and Nelarabin in T-Lin) in molecular failure (MolFail) after C2 (group 2).
882 patients (pts) from 142 sites were included 2003-2021 (table 1). 5% were withdrawn early and therefore not evaluable. The median age was 68 (56-86) yrs. 61% were older than 65 yrs. B-Lin-ALL was present in 68%; 7% of B-Lin-ALL (N=45) had a KMT2A-rearrangement. 19% and 9% had immature subtypes, pro-B and early T-ALL resp.. 50% had at least one comorbidity according to the Charlson-Score (ChS); 11% had a score ≥3; 28% had an BMI≥30 mg/m2. Group 2 vs group 1 had a significantly lower median age (66 vs 68 yrs; p=.001) and a higher proportion of T-ALL (21% vs 14%; p=.0003).
In 841 pts the CR rate after induction was 73% with 14% early death (ED) and 13% failure. The ED rate was lower in group 2 vs group 1 (9% vs 15%; p=.04). CR rates were 76%, 73% and 63% in three age groups (56-65, 66-75, ≥76 yrs) with ED rates of 9%, 14% and 26% resp. (p<.0001). CR rates were significantly lower in pts with WBC ≥30.000/µl (table 1). BMI (≥30 kg/m2) was not associated to CR/ED but ED rates were correlated to ChS with a high ED rate (26%) in those with ChS ≥3 (table 1).
MRD response data were available in 163, 239 and 173 pts after induction II, C1 and C2 resp. The molecular CR (MolCR)/ MolFail rates were 41%/52%, 57%/38% and 64%/30% resp. The remaining pts had intermediate MRD (MolIMR). MolCR rates were similar in B-Lin only (43%/58%/64%).
With a median follow up of 2.7 yrs overall survival (OS) in 841 pts at 3, 5 and 10 yrs was 36%, 28% and 22% resp. The 3y remission probability was 37%. Mortality in CR was 5% (3%, 6% and 7% by age) and 12% of the pts were withdrawn from protocol. OS strongly correlated to age and was very poor in pts older than 75 yrs (7% at 3 yrs; table 1). Only 8% (N=51) of all CR pts received an allogeneic stem cell transplantation (SCT) in CR1 (N=20 ≥65 yrs) but the SCT-rate increased in group 2 vs group 1 (9% vs 5%). 3y-OS after SCT in this selected group (N=51) was 56%.
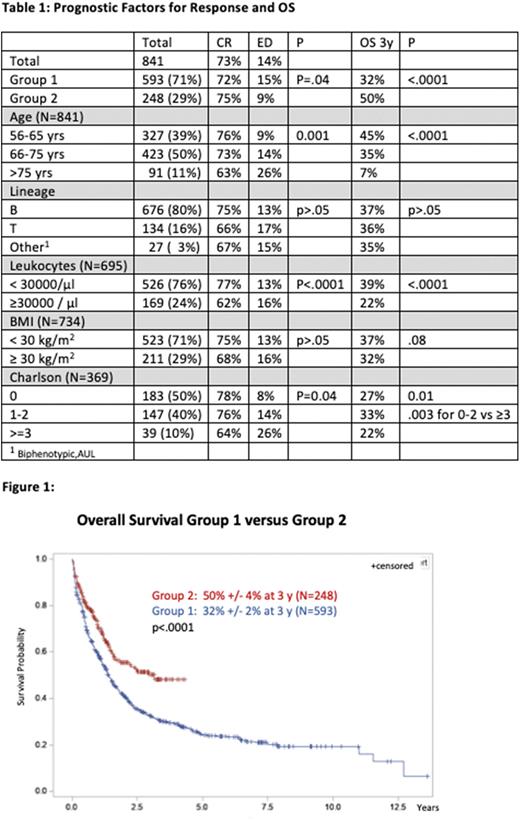
OS was significantly superior in group 2 vs group 1 (50% vs 32%; p<0.001) (figure 1). This improvement occurred mainly in the younger age group (56-65 yrs): 62% vs 38% 3y OS in group 2 vs group 1 resp. (p<0.0001) with no significant improvement in pts older than 65 yrs. WBC and ChS≥3 were associated with poorer OS (table 1). MRD response had a significant impact on OS at all timepoints. Pts with MolCR after C1 had a 3 yr OS of 80% vs 52% for MolFail and 62% for MolIMR (p=.0004). Interestingly the 3y-OS of 52 MolFail pts tended to be superior in group 2 (74% vs 46%;p>.05). The CR rate and OS of MLL-rearranged cases was 62% and 19% resp.
With this age-adapted, pediatric-based regimen a reasonable CR rate was achieved up to 75 yrs. Pt numbers were small and OS very poor in pts >75 yrs. MolCR rates were overall lower compared to more intensive protocols in younger pts. Thus, treatment modification based on MolFail is a promising approach and might produce benefits in many older pts. The optimal time-point for treatment change remains to be defined. Together with more intensive consolidation including PEG-ASP this modification likely contributed to the improved outcome with 62% OS in the youngest cohort (56-65 yrs) in group 2. In all older pts, but specifically in those >65-75 yrs or with multiple comorbidities, alternative protocols with targeted therapies and further step-wise reduction of chemotherapy require prospective exploration in clinical trials with the major goal to reduce ED rate and to improve MRD response.
Disclosures
Goekbuget:Gilead/Kite: Other: Research funding (institution); invited talk; advisory board; Erytech: Other: Advisory board; Cellestia: Other: Advisory board; Incyte: Other: Research funding (institution); advisory board; Amgen: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Pfizer: Consultancy, Other: Research funding (institution); invited speaker; advisory board; MorphoSys: Other: Advisory board; Jazz Pharmaceuticals: Other: Research funding (institution); advisory board; AstraZeneca: Other: Invited talk for company-sponsored symposia (with honoraria); AbbVie: Other: Research funding (institution); Novartis: Other: Research funding (institution); advisory board; Servier: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Clinigen: Other: Advisory board. Viardot:Kite Gilead: Consultancy, Honoraria, Other: Support for meeting attendance; Novartis: Consultancy, Honoraria, Other: Support for meeting attendance; BMS: Consultancy, Honoraria, Other: Support for meeting attendance; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Other: Support for meeting attendance; Janssen-Cilag: Honoraria, Other: Support for meeting attendance; Astra Zeneca: Honoraria, Other: Support for meeting attendance; Incyte: Consultancy, Other: Support for meeting attendance. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Teichmann:Boehringer-Ingelheim: Honoraria; AOP: Honoraria; Sobi: Consultancy, Honoraria; Pfizer: Consultancy; BMS: Consultancy, Honoraria; Astellas: Consultancy; BeiGene: Other; Gilead: Other: n. Alsdorf:Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants; Biontech: Other: Travel Grants, Research Funding. Schwartz:Amgen: Honoraria, Other: Advisory Board; CSI GmbH (Amgen/Jazz Pharmaceuticals): Speakers Bureau. Stelljes:MSD: Consultancy, Honoraria; Jazz: Honoraria; Kite: Consultancy, Honoraria; MSD Sharp & Dohme: Consultancy; Medac: Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Alakel:Pfizer: Consultancy, Honoraria. Hoelzer:Amgen: Speakers Bureau; Paladin Labs: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.